
Fat Metabolism
last authored:
last reviewed:
Introduction
Intestinal Absorption, Processing, and Secretion
Following digestion int the small intestine lumen, bile salts combine with FFAs, cholesterol, and 2-monoacylglycerol to form mixed micelles, which are disc shaped and 4-6 nm across. These micelles diffuse into the unstirred water layer and dissociate in the low pH found there. The lipids are then absorbed by the brush border of the intestinal mucosa. Short-and medium-chain FAs do not require mixed micelles to be absorbed.
TAG biosynthesis takes place in the sER, involving many different enzymes, and produces TAGs, cholesterol esters, and phospholipids. Short- and medium-chain FAs are released directly into the portal circulation.
Newly synthesized fats are very hydrophobic and aggregate. These aggregates, or chylomicrons, are surrounded by phospholipids, unesterified cholesterol, and apolipoprotein B-48. These particles give fat-rich lymph, or chyle, a milky appearance. Chylomicrons are released into lacteals and flow to the thoracic duct, into the left subclavian vein, and into the blood, where they transport TAG to peripheral tissues.
Lipid Absorption in the Periphery
Lipids are absorbed by various tissues in the periphery using recognition of specific proteins on different lipoproteins.
All tissues contain LDL receptor, recognizing Apo B-100 or Apo E on LDL for taking up cholesterol.
Liver, brain, and placenta also contain LRP (LDL-Receptor-related Protein) that recognizes Apo E on chylomicrons and VLDL remnants.
Scavenger receptors SR-A1 and -A2 on macrophages take up oxidized LDL.
Fatty Acid Synthesis
Fatty acids are used to build more compex molecules, as structural components, and as an energy source, particularly in liver and muscle.
Carbohydrates, proteins, and other molecules in excess of the body's needs can be converted into fatty acids for storage as TAGs. Fatty acid synthesis occurs primarily in the liver, in lactating mammary glands, and to a lesser extend in adiopose tissue.
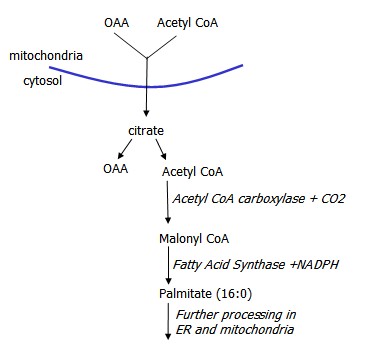
Fatty acids can be synthesized from Acetyl CoA, derived from the TCA cycle in reverse. Citrate is converted into OAA while generating Acetyl CoA. OAA is converted to malate, which then can become pyruvate.
Acetyl CoA acted on by Acetyl CoA Carboxylase to produce Malonyl CoA, which is then acted on by Fatty Acid Synthase (FAS), a multi-enzyme complex, that sequentially adds 2 carbon units, yielding palmitate. This process requires NADPH, provided by the pentose phosphate pathway.
Malonyl CoA accumulation inhibits fatty acid beta-oxidation.
Fatty acids can then go on to be used in TAG synthesis.
Conversion of Acetyl CoA to Malonyl CoA is increased by insulin and TCA intermediate citrate, and decreased by glucagon and epinephrine and palmitoyl CoA, downstream in the process.
Hormonal Derivitaves of Essential Fatty Acids
Essential fatty acids include linoleic acid (18:2) and alpha-linolenic acid (18:3).
Seed oils are used to produce arachidonic acid, which is then used to produce thromboxanes and prostaglandins.
Fish oils are used to produce eicosapentaenoic acid (EPA), which can also be used to produce other thromboxanes and prostaglandins.
TAG Synthesis
TAGs are comprised of a glycerol backbone and fatty acids. TAG synthesis occurs in intestinal cells following lipid digestion, in preparation for secretion as chylomicrons.
FFAs are first bound to CoA in an energy dependent process. FA-CoAs can then be attached to the2-MAG backbone by acyltransferases.
Glycerol must first be phosphorylated in order to form TAGs. This occurs in the liver and adipose tissue in one of two ways.
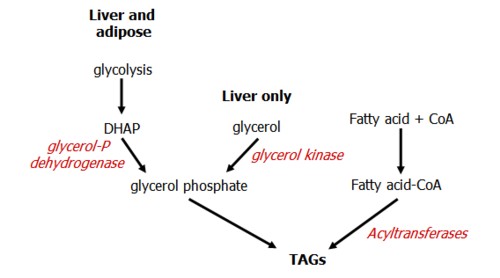
The liver and adipose tissue can convert glucose into glycerol 3-P through action of glycerol-P dehydrogenase on DHAP, in the glycolysis pathway.
The liver can also phosphorylate glycerol directly, hence once liberated in the blood (by hormone-sensitive lipase) it returns to the liver for processing.
Glycerol phosphate combines with fatty acyl CoA from fatty acid synthesis to yield a 1,2 diglyceride and finally a triglyceride.
TAG Fate
Little fat is stored in the liver, instread being combined with apoproteins and other lipids to form VLDLs that are released into the blood.
Adipose stores of TAG form the body's major source of stored energy. Stores are released following binding of hormones, especially epinephrine, transduction through the cAMP pathway, and activation of hormone-sensitive lipase.
Lipids as Fuel
After a meal, chylomicrons containing TAG and apo C-II are acted upon by lipoprotein lipase, located in the capillaries of muscle and adipose tissue and activated by insulin. FFAs can be taken up directly or transported on albumin.
During fasting, proteins are used as an energy source following glycogen depletion, generating carbon skeletons following deamination that can be used at various points in the TCA Cycle.
Levels of total urinary nitrogen, a measure of deamination, peak early during fasting and fall of during the proceeding weeks. In order to maintain levels, TAG stores are therefore mobilized.
Release of TAG Stores in Adipose Tissue
In the fat, a high epinephrine/insulin ratio activates hormone-sensitive lipase via cAMP and PKA, liberating FFAs and glycerol to the blood.
Glycerol cannot be metabolized in adipose tissue, and so it must return to the liver to be metabolized by glycerol kinase for future use in TAG synthesis. It can also be used as a substrate for gluconeogenesis.
FFAs diffuse through the plasma membrane and bind to albumin, where they are transported to the tissues and are oxidized for their energy. Active transport across membranes is mediated by fatty acid binding protein. FAs can also be transported to the liver, where they can contribute to the formation of ketone bodies.
Fatty Acid Oxidation
Fatty acid oxidation is used as an important energy source for tissues including skeletal and heart muscle. Fatty acid oxidation occurs in the mitochondrion, while fatty acid biosynthesis occurs in the cytoplasm.
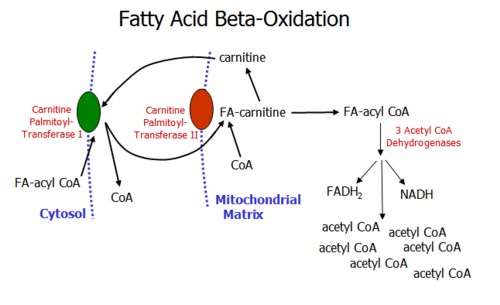
Accumulation of malonyl CoA, an intermediate of fatty acid synthesis, inhibits beta- oxidation of fatty acids.
Fatty acid oxidation occurs in the mitochondrial matrix through a number of steps and requires carnitine. Through the beta-oxidation spiral, the fatty acid chain is sequentially shortened by 2 carbons through the action of three acylCoA dehydrogenases to produce acetyl CoA molecules which enter the TCA cycle.
Oxidation of FAs with odd numbers of C produce a 3 carbon structure, propionyl CoA. This is oxidized in a three step process which involves L-methylmalonyl CoA and succinyl CoA, and requires Vitamin B12.
Beta oxidation of FAs produces energy from FADH2, NADH, and Acetyl CoA. The energy output is high: for each palmitoyl CoA, 131 ATPs are produced.
Fatty Acid Oxidation Defects
FA oxidation can be blocked by failure to enter the mitochondria, usually due to some defect with carnitine.
Genetic defects may also be present in one of the three acylCoA dehydrogenase enzymes, with the medium chain deficiency being the most common. This results in the excretion of dicarboxylic acids and leads to increasing use of glucose and often profound hypoglycemia.
Vitamin B12 deficiency leads to urine buildup of methylmalonic acidemia and aciduria, leading to metabolic acidosis and developmental disability.
Zellweger Syndrome occurs due to a loss of functional peroxisomes, which oxidize very long fatty acids. Their accumulation leads to death within 6 months of age.
