
Fats (Lipids)
last authored:
last reviewed:
Introduction
Triglyceride (TAG)
Fats are important macromolecules which are relatively insoluble in water.
The most important types of fats are triglycerides, phospholipids, and steroids. Triglycerides have three fatty acid tails attached to a glycerol backbone. Membrane fats are called phospholipids due to their modification with a negative phosphate group. Steroids such as cholesterol are quite differently shaped that fatty acids, but are classified as lipids due to their insolubility.
Fat intake is an important consideration in nutrition. Lipids are the primary source of energy for all tissues (save the brain between meals) and play an important role in energy storage as fat.
Adults eat between 60-150g of lipids/day, 90% of which is TAG. The rest is cholesterol, phospholipids, and free (unesterified) fatty acids.
Lipid digestion occurs throughout the GI tract, with the bulk occurring in the small intestine. Lipid uptake is mediated by bile. This is followed by transport and processing in the liver. Lipid transport in the circulation is mediated by lipoproteins such as HDL and LDL.
Lipid surface area is increased by breaking them down to facilitate their interaction with amphipathic molecules, such as bile, and enzymes. Large hydrophobic molecules are degraded into their smallest units for transport into cells. Large lipid particles are transported in stabilized particles, while small hydrophobic molecules are bound to hydrophilic proteins inside cells (ie fatty acid binding proteins) and in blood (albumin).
After a meal, chylomicrons containing TAG and apo C-II are acted upon by lipoprotein lipase, located in the capillaries of muscle and adipose tissue and activated by insulin. FFAs can be taken up directly or transported on albumin.
Dyslipidemias can be caused by defects in lipases, apo proteins, their receptors, or other causes.
Atherosclerosis results from the accumulation of lipids in blood vessel walls, leading to angina, acute coronary syndromes, renal disease, strokes, peripheral vascular disease, and other serious health threats.
Types of Fat
Fat structure is determined by length, degree of saturation, and the position of the first double bond.
- mono- and poly-unsaturated
- saturated
- trans
- cholesterol
Mono- and Polyunsaturated Fats
Monounsaturated fats are ok but not as good as the polys. Polyunsaturated fatty acids (PUFA) contain more than one double bond. Some PUFAs appear strongly beneficial in heart disease and perhaps other conditions (ref). The more unsaturated fats in your cells, the more pliable and dynamic their membranes are.
omega-3 fatty acids suppress cardiac arrhythmias, lower serum TAG, reduce thrombosis, and substantially reduce the risk of cardiovascular mortality. However, they do not appear to lower LDL or increase HDL in people.
- α-linolenic acid (ALA) is found in plants such as flax, soybean, and others
- docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are found in fish oils
- omega-3 derived eicosanoids are less active and antiaggregatory
omega-6 fatty acids
- linoleic acid
- gamma-linolenic acid
- arachidonic acid and eicosanoids
Saturated Fats
Saturated fats primarily come from animal sources and can have troubling health effects. They have two hydrogen atoms for each carbon in their tails, allowing them to pack closely together and leading to their solidity at room temperature.
Trans Fats
Trans fats are unsaturated fats which have been chemically modified to give them a longer shelf life, improve their taste and consistency, and increase their stability while cooking. However, this modification has serious health risks, with almost 30% of heart disease in the developed world appearing to be caused by trans fats (Mozaffarian et al, NEJM 2006). This showed:
- "a 2% increase in energy intake from trans fatty acids was associated wirh a 23% increase in the incidence of coronary artery disease"
- perhaps 20% of CHD events in the US are preventable by avoiding trans fats and replacing with omega-3 fatty acids.
Proposed Mechanisms of Action
act on hepatocyte, endothelial cells, adipocytes, and macrophages.
beware that less than 0.5 g can be called 0 grams - avoid hydrogenated fats
Cholesterol
Cholesterol, a steroid alcohol, performs a number of important functions in the body:
- a structural component of the cell membrane
- precursor of bile salts
- framework for steroid hormones, and vitamin D
As such, the cell needs a constant supply of cholesterol. Cholesterol biosynthesis and regulation is primarily mediated by the liver. From 0.2-0.5 g of cholesterol is ingested daily, and 0.8-1.0 g/day is synthesized.
Cholesterol is transported to cells on LDL particles, which contain Apo B-100 that bind to LDL receptors. Cholesterol is then used, or esterified and stored by activity of the enzyme ACAT.
The transport of cholesterol according to need is not precise, and over time there is a gradual deposition of cholesterol in the tissues, particularly the endothelium.
It can also be a troublesome molecule. In situations of dyslipidemia, cholesterol can accumulate on blood vessel walls and contribute to atherosclerosis. HDL and LDL are transport carriers for cholesterol in the blood.
Structure of Cholesterol
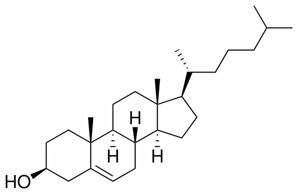 Cholesterol is very hydrophobic due to its four fused rings and its 8-carbon branched hydrocarbon chain. Steroids
that have an 8-10 Carbon chain and a hydroxyl group are called sterols.
Cholesterol is very hydrophobic due to its four fused rings and its 8-carbon branched hydrocarbon chain. Steroids
that have an 8-10 Carbon chain and a hydroxyl group are called sterols.
Most plasma cholesterol is esterified, with a FA attached to the C-3 hydroxyl group. Cholesterol esters are not normally found in membranes, and because of their hydrophobicity they must be transported either in lipoproteins or in bile salts.
Plant sterols are poorly absorbed and are actively transported back to the intestine. They appear to bring with them cholesterol molecules, leading to the use of plant sterol esters (ie margarine) to reduce cholesterol levels.
Cholesterol Synthesis
Cholesterol is made in almost every tissue, though the liver, intestine, adrenal cortex and reproductive organs make the largest amounts. All the carbon atoms are provided by acetate, and NADPH is the reducing agent. It is driven by hydrolysis of the thioester bond of acetyl CoA and ATP, and occurs in the cytoplasm. Enzymes are both in the cytoplasm and on the ER membrane.
Three acetyl CoA molecules combine to form HMG CoA, with 6 carbons. Next, HMG-Coa is reduced to mevalonic acid by HMG CoA reductase, the rate-limiting step of cholesterol synthesis. This reaction releases CoA, making the reaction irreversible. From there many steps occur to lead to cholesterol synthesis.
Regulation of Plasma Cholesterol
HMG CoA Reductase is regulated at the transcriptional level, by phosphorylation (inactivates), or by degradation.
- Cholesterol levels affect HMG CoA reductase mRNA and protein stability
- transcription of its gene is increased by Sterol Regulatory Element-Binding Protein (SREBP is usually associated with the ER membrane.
- Insulin upregulates HMG CoA reductase transcription, while glucagon has the opposite effect.
- Statins are structural analogues of HMG CoA, and are reversible competitive inhibitors of HPG CoA Reductase.
There are a number of ways to decrease plasma colesterol levels.
Dietary cholesterol has minimal effect on plasma cholesterol levels. N-6 PUFAs can reduce LDL levels, as does the Mediterranean diet, rich in monounsaturated fat. N-3 PUFAs appear not to have an effect.
Maximally decreasing dietary cholesterol and saturated fats can reduce plasma levels by up to 20%.
- dietary intervention - decrease cholesterol and SFAs, increase PUFAs, and add fibre
- statins - HMC CoA- reductase inhibitors
- ezetimibe - inhibits cholesterol uptake in the gut, reducing serum cholesterol by up to 20%
- vytorin - combination ezetimibe and simvastatin
Cholesterol Degradation
As the ring structure of cholesterol cannot be broken down, it is converted into bile acids and salts, or excreted in the bile. Some of the cholesterol is modified by fecal bacteria to form coprostanol and cholestanol.
Function of Fats
Fat as Fuel
One gram of fat produces 9 calories when oxidized, more than any other molecule.
During fasting, proteins are used as an energy source following glycogen depletion, generating carbon skeletons following deamination that can be used at various points in the TCA Cycle.
Levels of total urinary nitrogen, a measure of deamination, peak early during fasting and fall of during the proceeding weeks. In order to maintain levels, TAG stores are therefore mobilized. Lipid degradation for use in energy occurs through beta-oxidation. Ketone bodies can result.
In the fat, insulin and epinephrine activates hormone-sensitive lipase via cAMP and PKA, liberating FFAs and glycerol to the blood.
Glycerol cannot be phosphorylated in adipose tissue, and so it must return to the liver to be metabolized.
Fatty acid and TAG may also be synthesized in some tissues.
Steroids alter fatty acid metabolism by preventing phospholipase A2 activity.
Other Functions
Important fat derivates include the eicosanoids, precursors of prostaglandins, leukotrienes, and thromboxanes. Fats are also used as precursors for various hormones. Because lipids are hydrophobic, they tend to be compartmentalized within the body, in cell membranes, as droplets of TAG inside adipocytes, and associated with protein (ie lipoproteins) during plasma transport.
Resources and References
Ballantyne CM. Am J Cardiol 1998;82:3Q-12Q
Can J Cardiol 2006;22:913-26
- risk categories and treatment strategies
