Gluconeogenesis
Gluconeogenesis is used as a source of blood glucose during the fasting state, peaking around two days. Levels begin to rise 4 hours after.
While all tissues use glycolysis as an oxidative source of energy, the liver and kidney are the major sites of gluconeogenesis. Of the glucose produced, 80% comes from the liver and 20% from the kidney. Kidney gluconeogenesis is coupled to ammonia production.
From Pyruvate to Glucose
Pyruvate, residing in the mitochondria, cannot be transported across the mitochondrial membrane itself. Instead, it is carboxylated to OAA, which is temporarily converted to malate for transport across the mitochondrial membrane. Once in the cytosol, malate becomes OAA again, which is then converted to phosphoenolpuruvate (PEP) in the glycolytic pathway.
All glycolytic reactions are reversible save three, which require specific enzymes. Glucose 6-phosphatase is located only in the liver.
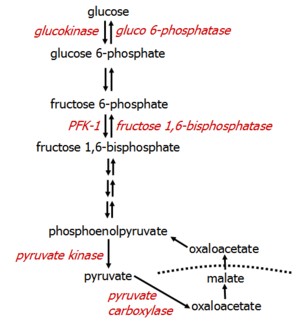
Beyond molecules of the TCA cycle, the most important molecules that enter gluconeogenesis include glycerol, lactate, and α-ketoacids.
Glycerol
Glycerol is released during TAG hydrolysis in adipose tissue and delivered to the liver.
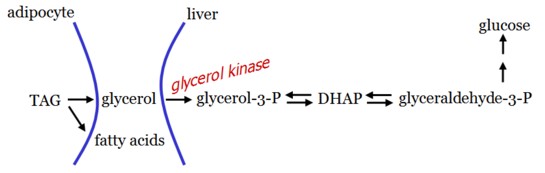
As adipocytes cannot phosphorylate glycerol, gluconeogenesis from glycerol can only occur in the liver.
Lactate
Lactate enters the blood from cells lacking mitochondria, such as RBCs, or by exercising muscle. Lactate returns to the liver, where it is reconverted to glucose for re-release into the blood in the Cori Cycle.
Protein-Dependent Gluconeogenesis
Urine excretion of urea is low during the fed state but increases dramatically 12 h after fasting, decreasing to fed state levels after a few weeks, providing a measure of increased protein degradation.
Amino acids can enter the TCA cycle at various points, including alpha-ketoglutarate, succinyl CoA, fumarate, and oxaloacetate. They can also be converted into puruvate and acetyl CoA. From there, oxaloacetate can be converted into PEP and returned to glucose.
Control of Gluconeogenesis
The ratio of insulin:glucagon regulates both glycolysis and gluconeogenesis.
Fructose 1,6-bisphosphatase is an important control point for gluconeogensis, and inhibited by high levels of fructose 2,6-bisphosphate, a potent activator of PFK-1 in glycolysis.
The insulin/glucagon ratio plays an important role in mediating F 2,6-bisP levels.
