
Acute Coronary Syndrome
last authored: Dec 2010, Sean Doran
last reviewed: Jan 2011, David LaPierre
Introduction
Acute coronary syndrome (ACS) describes three different but related conditions in which heart tissue dies - a heart attack - or is at very high risk of death. Each share a common pathological process of insufficient oxygen and nutrients reaching the heart. Myocardial infarction (MI) refers to the death (infarction) of heart cells, while in unstable angina cell death has not yet occurred, but is at very high risk of occurring.
MI is a leading cause of morbidity and death in affluent countries, and increasingly around the world. In Canada, 35% of deaths are due to ACS. Nearly 10% of MIs occur in people under the age of 40, and almost half affect people under 65% (ref).
Heart attacks can be of two types :
- non-ST elevation myocardial infarction (NSTEMI)
- ST elevation myocardial infarction (STEMI)
Again, the third condition described as ACS is unstable angina.
ACS can result in immediate death if damage is severe enough. Patients who survive can be left with significant loss of heart function. However, if diagnosis and intervention are prompt and effective enough, lasting damage can be slight, or not at all.
The Case of Fred Z.
Fred is a 68 year-old man who develops nausea and a vague chest discomfort after supper. He blames it on indigestion, but half and hour later his chest pain becomes much worse and he collapses. His wife drives him to the hospital.
- What questions do you ask?
- What immediate treatments do you offer?
- What tests do you do?
Causes and Risk Factors
Acute coronary syndromes typically result from a disruption of an atherosclerotic plaque in a blood vessel.
Risk factors for ACS therefore are those of atherosclerosis, and include:
- increased age
- hypertension
- smoking
- diabetes
- high plasma LDL and low HDL
- family history and genetic polymorphisms: 9p21, when homozygotic, increases the risk of CHD by 30-40% (McPherson et al, Science, 2007)
- infection and inflammation
- metabolic syndrome
- sedentary lifestyle
Less common etiologies include any instance where there is significantly increased demand on the heart coupled with poor myocardial perfusion (i.e. trauma with massive blood loss, anemia, infection, etc.). These less common cases are not the focus of this discussion.
Pathophysiology
main article: atherosclerosis
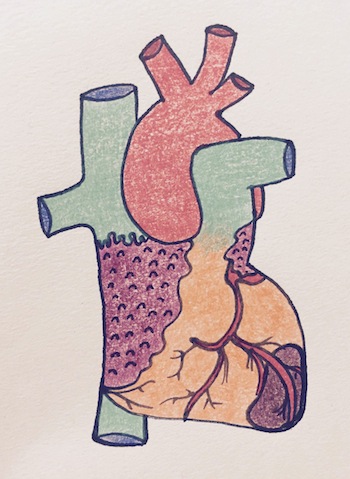
Myocardial infarction, by Shannon Young
More than 90% of ACS results from disruption of an atherosclerotic plaque, with subsequent acute inflammation, endothelial damage, platelet aggregation and thrombus formation. This leads to severe or complete occlusion of the vessel. The other 10% are due to intense and prolonged vasospasm, sometimes related to cocaine abuse, emboli, or idiopathic.
Occlusion can lead to unstable angina (ischemia without myonecrosis) and/or myocardial infarction (ischemia resulting in myonecrosis or infarct). In ST elevation, total coronary artery occulusion has occurred, leading to necrosis.
Note, it is the acute inflammatory process that precipitates ACS, not the severity of underlying coronary artery disease prior to the event. Furthermore, the inflammatory process often leads to increased central and sympathetic nervous system activation which results in vasomotor hyper-reactivity and coronary vasospasm - exacerbating the insult to myocardial tissue.
Inadequate tissue perfusion results in myocardial ischemia when the oxygen demand of myocardial tissue exceeds the supply. Oxygen consumption at the level of myocardial tissue is dependent on four physiological parameters: heart rate, afterload, contractility, and wall tension.
- Gross image of LV post-MI (new window)
- Cross section of recent transmural MI (new window)
In other cases, vasoconstriction or vessel spasm, occurring in epicardial or subendocardial vessels, will lead to ischemia.
Arterial inflammation, caused by infection or other causes, can also lead to plaque growth and disruption.
- plaque disruption
- tissue infarction
- territories
Plaque disruption
Most often, ACS results from abrupt disruption of a previously only partially stenosed plaque, which undergoes any of the following:
- rupture/fissuring, exposing highly thrombogenic plaque constituents
- erosion or ulceration, exposing thrombogenic basement membrane
- hemorrhage into the plaque, expanding its volume
Frequently, within minutes, the lumen is completely blocked.
Those that undergo abrupt disruption often are only mildly to moderately stenosed. A rather large number of asymptomatic adults therefore are at risk of ACS. It is also increasingly clear that plaque disruption and thrombosis can occur in silent repetitive waves.
The triggering events are complex and poorly understood, but include plaque characteristics, blood pressure, and platelet reactivity. Adrenergic increases in blood pressure or local vasospasm can be lethal. Awakening and arising leads to the peak incidence of MI occurring between 6am-noon. Emotional stress can also contribute to plaque disruption, illustrated by the increase in sudden death following disasters.
Inflammation is also very important throughout the process of atherosclerosis. T cells produce TNF, IL-6, and IFN-gamma, stimulating endothelial cells and activating macrophages. Macrophage secretion of metalloproteinases weaken the fibrous cap and can lead to rupture.
Vasoconstriction can be mediated by adrenergic factors, platelet activation, and possible inflammatory mediators.
Tissue Infarction
Within seconds of vessel occlusion, aerobic glycolysis stops and lactic acid begins to build up. A striking loss of contractility occurs within 60 seconds of ischemia onset, which can precipitate heart failure long before infarction. Only severe ischemia lasting 20-40 minutes leads to cell death in the form of coagulation necrosis.
In unstable angina and NSTEMI, the vessel is usually not completely blocked, resulting in no or little necrosis. If the vessel is blocked, however, severe ischemia results in the significant necrosis accompanying STEMI.
Most MIs are transmural, in which necrosis extends through the ventricular wall. A wave front of cell death moves through the wall, and necrosis appears largely complete within 6 hours. Hearts with developed coronary arterial collaterals may take up to 12 hours to fully necrose.
The subendocardium is most susceptible to damage, and some MIs are limited to the inner half of the wall. Subendocardial infarctions can result from a thrombus which is lysed early after forming, but can also follow prolonged shock superimposed on nonruptured coronary stenosis.
Scarring is well advanced after 6 weeks.
Territories
main article: heart structure
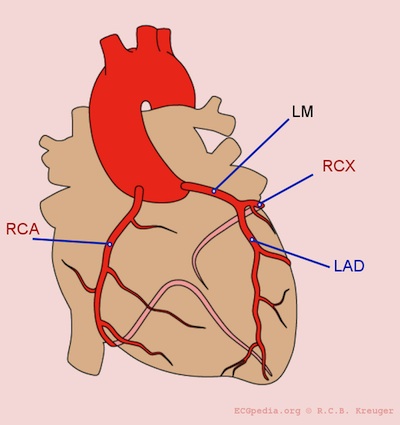
Coronary arteries, from ECGpedia
Nearly all transmural infarcts involve at least a portion of the left ventricle, including the septum. About 15-30% of those affecting the posterior wall extend into the right atrium. Atrial tissue can sometimes become infarcted with large posterior wall infarcts.
Left anterior descending (LAD) infarcts (40-50%) involve the anterior left ventricle near the apex, the anterior septum, and the apex circumferentially. These anterior MI's have a significant impact on cardiac output due to loss of contractility, leading to hypotension, tachycardia, and heart failure.
Left circumflex (LCX)/ramus circumflexus (RCX) infarcts (15-20%) involve the lateral wall of the left ventricle, except the apex.
Right cerebral artery (RCA) infarcts (30-40%) involve:
- the inferior/posterior wall of the left ventricle
- the posterior septum
- right ventricle
- SA and AV node, posterial left bundle branch
As the right ventricle is dependent on preload for cardiac output, hypotension is common, and should be treated with IV fluid boluses. Nitroglycerin should be used with extreme caution, given the risk of preload reduction.
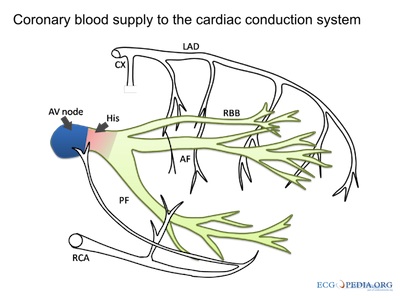
courtesy of ECGpedia.org
Disruption to the vasculature can also have a significant impact on heart rhythms, as described at right.
Signs and Symptoms
- history
- physical exam
History
Symptoms of ACS vary considerably, and can be vague, especially in women or people with diabetes.
Chest symptoms can include:
- pain
- heaviness
- pressure
- tightening
Radiation of discomfort can include:
- left arm or shoulder
- shoulder blades
- neck or jaw
- upper abdomen
Symptoms can arise or worsen with exertion.
Other symptoms can include:
- diaphoresis
- generalized weakness, fatigue, and dizziness
- dyspnea
- nausea, vomiting
- anxiety, or feelings of extreme unease
- new or unusual indigestion
- at least 15 minutes of ischemic chest pain at rest
- worsening of previously stable effort angina, ie frequency, severity, or duration
- history of recent (2 mo) onset of CCS class III or IV angina
Clinical characteristics that raise the likelihood of cardiac chest pain include:
- dull, pressure-like retrosternal chest discomfort
- duration approximately 2 to 5 minutes
- gradual onset
- reproducible/exacerbated with exertion
- pain not reproducible with palpation
In 10-15% of people, particuarly the elderly and those with diabetes, silent MIs are only discovered later by ECG changes, usually new Q waves.
Particular attention should be paid to risk factors for cardiovascular disease:
- male gender
- increasing age
- smoking history
- hypertension
- diabetes mellitus
- hypercholesterolemia
- family history of cardiovascular disease (first degree relatives, male < 55 y & female < 65 y)
- substance abuse (particularly cocaine abuse)
Physical Exam
When assessing a patient you suspect of having ACS, begin with an immediate and regular recording of the patient's vital signs.
The physical exam should focus on cardiovascular, respiratory, abdominal and neurological findings. The goal is to explore for other factors that may contribute to the patients symptoms and increase or decrease the likelihood of a diagnosis of ACS.
Cardiovascular exam:
Respiratory exam:
Neurological exam:
People can have a rapid, weak pulse and are often sweating profusely.
A useful way to remember factors that reduce the likelihood of chest pain being cardiac in origin is the "three P's":
- pleuritic
- positional
- reproducible by palpation
Signs of right ventricular infarction are as follows:
- jugular vein distension
- hypotension
- lung fields free
Investigations
The history and physical exam are crucial components of the assessment of any patient presenting with possible ACS. However, these should be obtained expediently as definitive diagnoses require electrocardiac studies and laboratory investigations.
- lab investigations
- diagnostic imaging
Lab Investigations
Serum markers of myocardial injury are also an important component of the ACS work-up. In an emergency department setting, these are most useful in patients with a non-diagnostic ECG yet the clinician retains a strong clinical suspicion for myocardial injury.
Fatally injured myocytes leak proteins into the blood. These include myoglobin, troponins, creatine kinase-MB, lactate dehydrogenase, and others. The preferred biomarkers are cardiac-specific, particularly the troponins. An absence of change in CK within 2 days essentially excludes MI. Reperfusion acceletates biopmarker presence due to enzyme washout.
For appropriate interpretation, serial measurements are usually obtained every 6 - 8 hours until levels have peaked.
troponin-T -I, and -C
- much more sensitive
- begins to rise 3-4 hours, peaking at 12-36 hours
- remain elevated 7-10 days after event
creatine kinase-MB
- former gold standard
- begins to rise with 2-4 hours ,peaks at 24 hours
- returns to normal within 72 hours, much shorter than the troponins
myoglobin
- cleared quickly by kidneys
cholesterol
BUN (kidneys)
Reperfusion
Before reperfusion: protrombin time (PT) and partial thromboplastin time (PTT) to ensure extrinsic and intrinsic clotting pathways are ok
Diagnostic Imaging
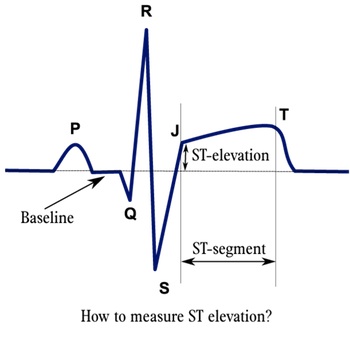
courtesy of ECGpedia
An electrocardiogram (ECG) is an essential component of the patient work up, and should be performed within 10 minutes of medical contact. Typical ECG findings of ACS include:
- T wave morphology changes (hyperacute and inversion)
- ST segment depressions (more likely ischemia) or elevations (more likely infarction)
- left bundle branch block
- QRS complex changes (new bundle branch block; evidence of Q waves - suggestive of old infarct)
- rhythm disturbances
continuous leads, from wikipedia
If available, comparison with an old ECG is particularly helpful. It is important to note that ECG changes are dynamic and thus, change over time. Therefore, in a patient in which ACS is highly suspected, serial ECGs are often appropriate.
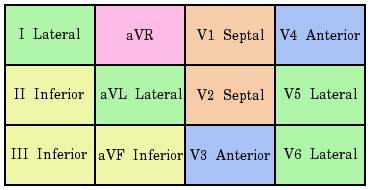
The location of a myocardial infarct can be determined from the pattern of morphological changes on the ECG.
A list of select examples is provided:
- anterior infarcts: pre-cordial leads (V1 - V4)
- septal infarcts: V1 and V2
- anteriolateral infarcts: V1 - V6 and I and aVL
- infarction involving the left anterior descending artery typically demonstrate ST segment elevation in leads V2 - V3 along with ST segment depression in lead II and III and/or aVF
- lateral infarcts: (I, aVL, V5 - V6)
- inferior infarctions: (II, III and aVF)
- isolated posterior infarctions are rare but can be demonstrated in accessory leads (V7 - V9)
ST depression
- troponin -ve - UA (45%)
- troponin +ve - NSTEMI (35%)
ST elevation
- STEMI (20%)
MI: biomarker evidence of necrosis (over 99th centile of upper reference limit) plus ischemic symptoms, new ischemic ECG changes, new pathological Qs, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality
Chest radiography should also be ordered - largely for the purpose of determining the appropriateness of certain therapies (e.g. fibrinolytic therapy). A chest X Ray is also valuable in determining if pulmonary congestion is evident.
Echocardiography is a useful test for the evaluation of patients with ACS. In particular, regional wall motion abnormalities contribute to the evidence supporting a diagnosis of acute MI. However, echocardiography usually takes some time to obtain and is somewhat dependent on operator technical skill and thus, less valuable in the acute setting. It is quite useful for an assessment of the extent of myocardial injury once the patient is stable.
Other diagnostic imaging modalities that may be used include myocardial scintigraphy and computed tomography.
Differential Diagnosis
Main article: chest pain
The differential diagnosis of chest pain is extensive and includes:
|
|
Treatments
If less than 12 h, with ST elevation or new LBBB, consider reperfusion. Within 90 minutes of presentation, PCI is preferable. Reperfusion can salvage tissue and limit infarction, as cell death takes 6-12 hours to fully occur. The first 3-4 hours are critical. Reperfusion within the first 15-20 minutes can prevent all necrosis. The old saying "time is myocardium" serves as a reminder that the longer the delay to reperfusion (when appropriate) the more extensive the myocardial damage will be.
- acute
- fibrinolytics
- catheterization
- chronic treatment
Once the clinician suspects acute coronary syndrome, a number of treatments (barring contraindications) should be initiated - beginning with an assessment of the patients airway, breathing and circulation.
Bedrest and telemetry
Acute treatment for suspected ACS include (pneumonic MONA)
- oxygen
- nitroglycerin (not if a right-sided MI is suspected, as cardiac output becomes very dependent on preload)
- aspirin
- morphine
Treatment with the following drugs should also be considered (with regard to vital signs, co-morbidities, etc.):
- antiplatelet agents - both ASA and clopidogrel
- anticoagulants - low molecular weight heparin, or heparin
- beta-blockers - if/once hemodynamically stable
- nitrates - sublingually or IV
- IV fluid resuscitation if hypotensive
- vasopressor support if cardiogenic shock is present
- angiotensin-converting enzyme inhibitors should be started within 24 hours
- statin should be started within 24 hours
Fibrinolytics
Fibrinolytics may be given within 12 hours of onset of symptoms, though aim for a door to needle time below 30 minutes
fibrinolytic absolute indications
- prior intracranial bleed
- head or facial trauma within 3 mo
- known cerebral vascular lesion
- intracranial malignancy
- ischemic stroke <3 months
- active bleeding
- suspected aortic dissection
fibrinolytic relative indications
- severe hypertension (180/110)
- current anticoagulation
- noncompressible vascular punctures
- major surgery within 6-8 weeks
- embolic stroke within 3-12 months
- pregnancy
- active peptic ulcers
Approximately half the risk of death with ULMWH.
enoxaparin is better
fondaparinux
- factor X inhibitor (i thnk)
- fixed dose, once daily sc administration
- chepaer than enoxaparin
- OASIS-5 showed much decreased risk of bleeding
- now preferred drug for anti-thrombin
- does not provent thrmbosis on lines, wires, etc
bivalirudin
- direct thrombin inhibitor
- short half life minimizes bleeding
- expensive
throbolytic therapy contraindicated in UA and NSTEMI
thrombolytics only work 45-50% of time
metaanalyisis shows field treatment with thrombolytics has a small benefit
Stroke predictors include old age, female, low predictors etc
Catheterization
Catheterization should be considered in patients with STEMI, or NSTEMI and:
- recurrent angina, despite therapy
- CHF or LV dysfunction
- hemodynamic isntability
- high TIMI score
- recurrent or sustained ventricular tachycardia
- dynamic ECG changes
- high-risk findings
- PCI within last 6 months
- repeated episodes of acute coronary syndrome
Pre-treat with anti-platelet therapy
Contraindications include:
- people beyond 12 hours
- severe peripheral vascular disease (difficult catheterization)
- chronic renal insufficiency (relative contraindication)
Chronic
Patients should be followed up regularly following MI.
Review should include:
- chest pain
- use of nitro spray
- shortness of breath
- palpitation
- pre-syncope
- neurological symptoms
- medication side effects
- adherence to medication plan
Risk factor management is incredibly important, including:
- hypertension
- dyslipidemia
- diabetes control
- smoking
- low salt, low fat diet
- exercise plan
Once stable, patients should be treated with:
- ASA
- ACE inhibitors
- clopidogrel (one month after bare metal stent, one year after drug-eluting stent)
- lipid-lowering agents
- beta-blockers
Consequences and Course
In-hospital mortality has declined from 30% in the 1960's to 10-13% today. Half of deaths occur within 1 hour of symptom onset.
Poor prognosis factors include advanced age, female gender, siebetes mellitus, and previous MI.
Nearly 75% of people have one or more complications, which include:
- heart failure, as a result of contractile dysfunction
- arrhythmias, including sinus bradycardia, heart block, tachycardias, PVCs, ventricular tachycardia, or ventricular fibrillation
- myocardial rupture, potentially leading to tamponade, shunting, or mitral regurgitation
- pericarditis
- infarct extension or expansion
- mural thrombus and potential emboli
- ventricular aneurysm
ventricular remodeling includes progressive changes in size, shape, and thickness of the heart, leading to initial benefit but later impairment.
Cardiogenic shock can arise 3 days later:
- acute temponade
- ischemic VSD
- severe mitral regurgitation
ACS patients have a high early recurrence rate which then levels off. Treat aggressively early.
Reperfusion is the best way to salvage ischemic heart tissue, but can lead to arrhythmias, contraction bands (supercontraction of already dead cells), or reperfusion injury. This is mediated, at least in part, by free radicals.
Not dead ischemic myocardium can remain stunned or hibernate.
in NS, one year mortality:
UA: 8%
STEMI: 17%
NSTEMI: 22%
- typically older people with multiple comorbidities, with widespread vascular disease
Resources and References
Andreoli et al. Cecil Essentials of Medicine 7th Ed. Saunders Elsevier. Philadelphia 2007.
Marx et al. Rosen's Emergency Medicine: Concepts and Clinical Practice 7th Ed. Mosby Elsevier. Philadelphia 2010.
Porter et al. The Merck Manual of Patient Symptoms: A Concise, Practical Guide to Etiology, Evaluation, and Treatment. Merck & Co., Inc. New Jersey 2008.
Tintanalli, JE Emergency Medicine (A Comprehensive Study Guide). McGraw-Hill Companies, Inc. USA 2004.
Topic Development
authors: Sean Doran, Caleb Zelenietz
reviewers: Una Doran, David LaPierre
