


Cellular respiration is the metabolism of carbohydrates and other molecules, using oxygen, and generating carbon dioxide during ATP synthesis.
The electron transport chain (ETC) occurs in the mitochondrion following generation of NADH and FADH2 from the TCA cycle. These coenzymes, when oxidized, can donate a pair of electrons to a set of specialized electron carriers.
The ETC is present in the inner mitochondrial membrane and is occurring continuously in all tissues containing mitochondria.
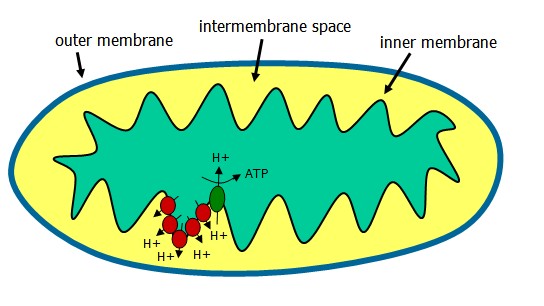
NADH is reduced by dehydrogenases that remove two hydrogens. Both electrons and one proton (a hydride ion) are transferred to the NAD+, forming NADH plus H+. The proton and hydride carried by NADH are next transferred to NADH dehydrogenase, complex 1 in the inner membrane. Coenzyme Q is complex II, and electrons are passed along the chain from complex II to the cytochromes of complex III and IV. Cytochromes, like hemoglobin, contain a heme group made up of a porphyrin ring and an atom of iron. The cytochrome iron is reversibly converted from its ferric (3+) to ferrous (2+) form as a normal part of its function.
Oxygen, an avid electron acceptor, is at the end of the electron transport chain. O2 + 4H+ -> 2 H20.
