
Renin-Angiotensin-Aldosterone System
The renin-angiotensin-aldosterone system (RAAS) is a key regulator of blood volume. It functions in multiple parallel pathways, allowing for powerfully adjustable control.
- Renin
- Angiotensin II
- Aldosterone
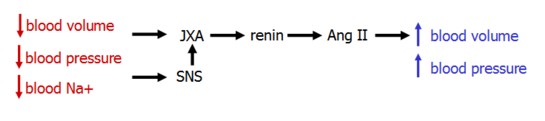
Renin
Renin is produced in the kidney's juxtaglomerular cells. It's secretion is stimulated by three imputs:
- norepinephrine released by symapthetic nerves in response to the systemic baroreceptor reflex
- decrease blood pressure, sensed by intrarenal baroreceptors in the JGA
- low [NaCl], sensed by the macula densa
Renin secretion is inhibited by ANP, which acts to increase GFR and sodium excretion.
Renin acts by cleaving angiotensinogen, produced in the liver, to form angiotensin I. Angiotensin I, in turn, is cleaved by angiotensin-converting enzyme (ACE) in the lung to form Ang II.
Angiotensin II
Angiotensin II is a powerful systemic vasoconstrictor that acts to increase both blood pressure and volume.
Elevated levels maintain blood pressure during hypovolemia, but can also increase blood pressure in euvolemic or hypervolemic states.
Site of Action |
Effect |
|---|---|
arterial SMCs |
vasoconstriction (NO production from bradykinin) |
adrenal gland |
aldosterone release |
SNS |
facilitates NA release |
kidney proximal tubule |
enhances Na+/H+ exchanger, leading to Na+ reabsorption |
brain |
stimulates thirst, ADH release |
heart |
inc. contractility and ventricular hypertrophy |
While Ang II constricts both afferent and efferent arterioles of the glomerulus, its dominant effect is efferent constriction.
This increases GFR and filtration fraction in the face of decreased renal blood flow is due to increased capillary osmotic pressure and decreased hydrostatic pressure.
Also, decreased vasa recta blood flow decreases urea washout from the medulllary interstitium, increasing the gradient for NaCl reabsorption in the thin ascending loop of Henle. This increases Na reabsorption.
The net effect is water and sodium retention.
Molecular Mechanisms of Action
The AT1 receptor is a GPCR that can induce many different cell signaling pathways. Akin to the binding of epinephrine to α2 receptors, AT1 activation in the proximal tubule inhibits adenylyl cyclase and cAMP production, which in turn increases the activity of the Na/H exchanger.
In the vasculature, activation of AT1 mimics epinephrine binding to α1 receptors by increasing levels of DAG and IP3.
Aldosterone
Aldosterone is a mineralcorticosteroid hormone synthesized from cholesterol in the adrenal cortex. It is active during times of volume contraction and low sodium concentration, working to increase plasma sodium levels, and thereby water.
While aldosterone's effects on sodium reabsorption are most prevalent in the principal cells of the distal tubule and collecting duct in the kidney, it also works on sweat glands and the colon to increase sodium uptake.
Aldosterone secretion is regulated in a number of ways:
- Ang II activity
- hypokalemia, sensed by the zona glomerulosa in the adrenal cortex
- ANP reduces aldosterone secretion
Aldosterone also works on muscle during times of hyperkalemia to increase Na/K pump activity to decrease ECF [K]. Increased K secretion in the cortical collecting tubule also reduces K concentration.
Intracellular Signaling of Aldosterone
Aldosterone binds to a cytoplasmic receptor and migrates to the nucleus, where it activates transcription of protein channels and pumps. It also activates proteins that increase existing channel and pump function, resulting in increased sodium reabsorption and potassium secretion. This includes apical Na and K channels, and the basal Na/K pump.
Aldosterone is involved with acid secretion, and icnreased aldosterone can cause metabolic alkalosis. Not sure how.

Loss of Aldosterone
Loss of adrenal function, as occurs in Addison's Disease, results in severe sodium depletion, ECF volume contraction, and circulatory insufficiency.